Update: Bill signed into law; effective immediately
October 11, 2021: Gov. Gavin Newsom on Friday, Oct. 8, signed CDA-sponsored legislation that gives California dentists permanent authority to provide influenza and COVID-19 vaccines and obtain the required state registration to process rapid COVID-19 tests in the dental office.
AB 526, authored by Assemblymember Jim Wood, DDS (D-Santa Rosa), overcomes two significant hurdles for California dentists who are managing patient care, particularly during the ongoing pandemic.
First, it allows them to independently prescribe and administer flu and COVID-19 vaccines in accordance with the federal vaccine schedules published by the Centers for Disease Control and Prevention. Second, it allows dentists to obtain the California Laboratory Field Services license to perform waived tests, such as rapid point-of-care COVID-19 tests, which would not only protect dentists and their staff but could help dentists comply with the COVID-19 testing requirement under the state public health order that took effect Aug. 23. (Update: The state's vaccinate-or-test mandate ended Sept. 17, 2022.)
The Legislature passed the bill Sept. 8 as an urgency statute, meaning the law would take effect immediately upon the governor's signing. He signed AB 526 and other legislation, including a COVID-19 recovery package, on Friday.
CDA will release an updated COVID-19 Laboratory Testing Toolkit early this week.
Bill codifies DCA waiver authorizing dentists to provide COVID-19 vaccines
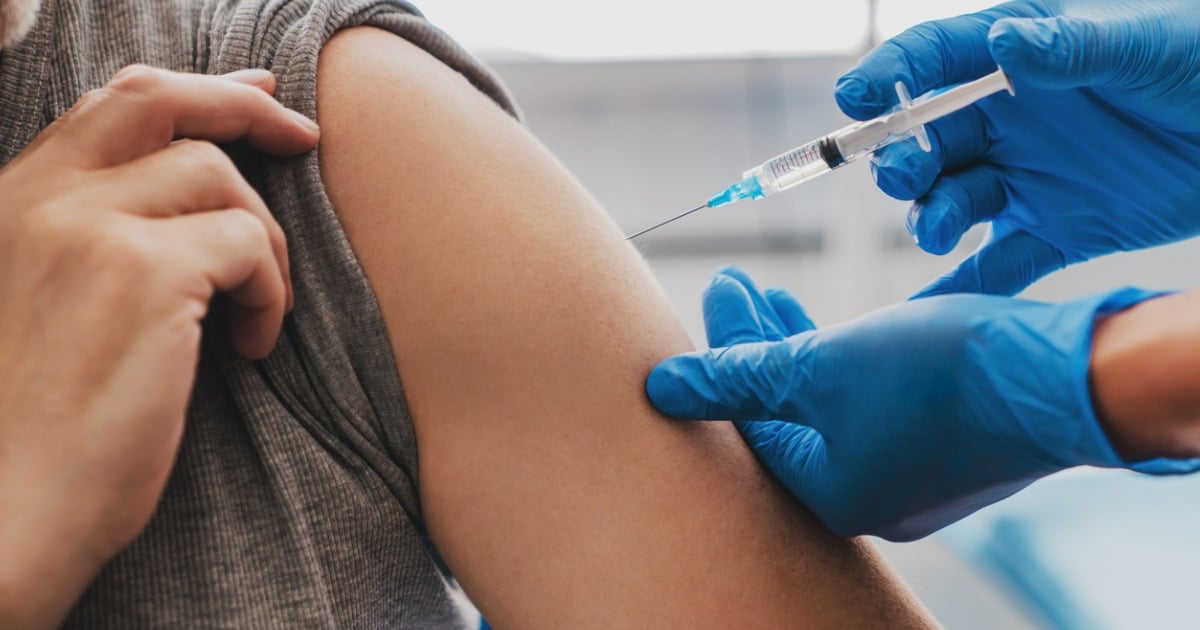
Many California dentists who completed the required Centers for Disease Control and Prevention trainings have been administering the COVID-19 vaccines on a temporary basis since the Department of Consumer Affairs in January issued a waiver to expand the number of eligible vaccinators during the public health emergency.
As CDA stated in a letter urging the Legislature’s support: “Dentists are well equipped to administer vaccines because they routinely provide injections to particularly sensitive areas of the mouth … and have extensive training in microbiology, autoimmune response, general anatomy, pharmacology and starting IVs.”
By codifying the DCA waiver and the federal PREP Act, AB 526 authorizes dentists to prescribe and administer FDA-approved or FDA-authorized flu and COVID-19 vaccines to individuals ages 3 and older. The bill also specifies that the CDC vaccine trainings will count toward a dentist’s continuing education requirements.
Bill opens path for dentists to conduct rapid COVID-19 tests in dental offices
AB 526 also allows dentists to obtain the appropriate state registration alongside their federal CLIA certificate so that conducting simple, reliable waived tests, such as rapid, point-of-care COVID-19 tests, falls within the scope of dental practice.
“Dentists need to be able to rapidly screen all patients before dental treatment to protect the dental team from COVID-19 exposure and to help direct asymptomatic, COVID-positive patients to appropriate care,” CDA wrote in a letter to the state Legislature.
Currently, dentists can obtain their CLIA Certificate of Waiver through the Centers for Medicare and Medicaid Services, which is required to conduct waived tests. Tests are classified as waived if the risk of misinterpreted results is low and if administration of the tests can be followed by adhering to the test manufacturer’s instructions.
However, before AB 526 became law, dentists were not eligible to obtain the required California Department of Public Health Laboratory Field Services registration because they were not on the list of individuals who can oversee the waived tests. That has changed. The new law aligns the California laboratory registration statute with federal law and allows dentists to register in the state as a “lab director” to conduct waived tests.
“Dentists have equivalent training to other providers who may register as a lab director and therefore should also be allowed to oversee the simple, low-complexity tests,” said CDA President Judee Tippett-Whyte, DDS. “With rapid, point-of-care COVID-19 tests coming into market, this legislation’s passage is all the more critical for immediate patient and employee screening to keep dental teams and patients safe.”
Now, dentists can conduct certain FDA-approved antigen COVID-19 tests approved by the FDA for self-administration to comply with the state public health order’s weekly employee COVID-19 testing mandate. More information is available in CDA’s COVID-19 Laboratory Testing Toolkit.