Sunstar Americas Inc. has expanded its voluntary nationwide recall on its prescription oral rinse.
A U.S. Food and Drug Administration news release states the company’s Paroex Chlorhexidine Gluconate Oral Rinse USP, 0.12% product could be contaminated with the bacteria Burkholderia lata and may result in oral infections and life-threatening infections, such as pneumonia and bacteremia for the most at-risk users.
The initial recall issued last October, stated that the affected products had expiration dates between June 30 and Sept. 30, 2022, but the FDA on Dec. 28, 2020, published an updated announcement expanding the expiration period from Dec. 31, 2020, to Sept. 30, 2022.
To date, 29 people have tested positive for Burkholderia lata infections from using the recalled product, according to the announcement.
The oral rinse, used primarily for treatment of gingivitis, is distributed nationwide to dental offices, dental distributors, pharmaceutical wholesalers, dental schools and pharmacies and packaged as:
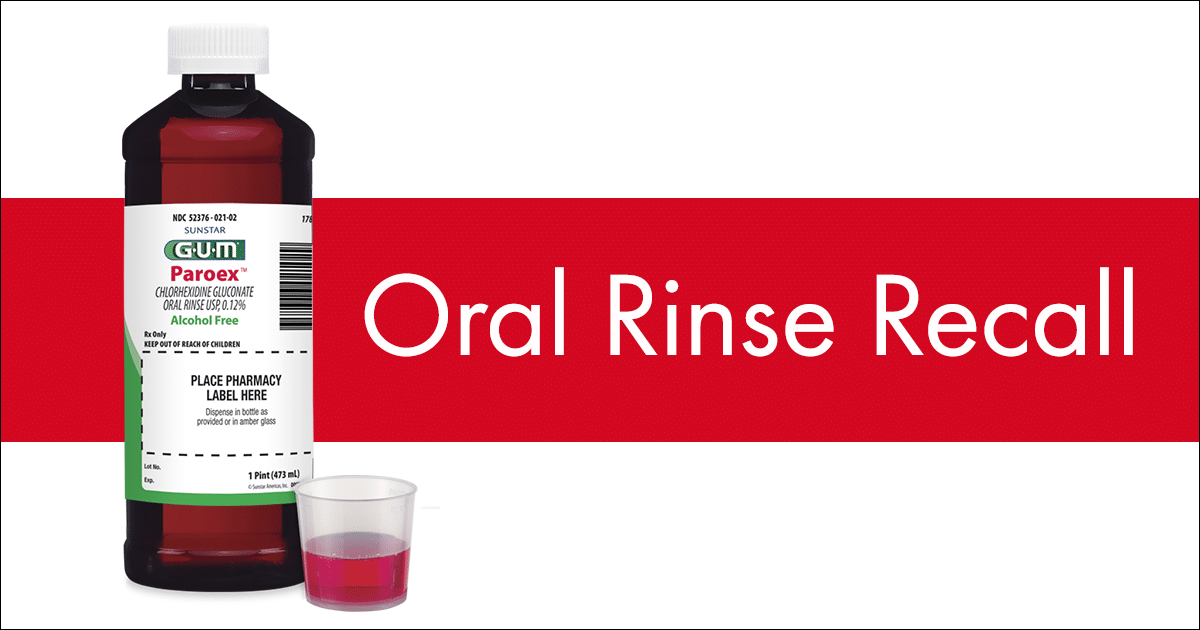
- 1789PGUM® Paroex® is distributed in cases with each containing six amber bottles of 16 fluid ounce (473 ml) chlorhexidine rinse. The bottle has a childproof cap and a 15 ml metered dosage cup, is safety sealed and is decorated with a multiple-panel wraparound label.
- 1788PGUM® Paroex® is distributed in cases with each containing 24 amber bottles of 4 fluid ounce (118.25 ml) chlorhexidine rinse. The bottle has a childproof cap, is safety sealed and is decorated with a multiple-panel wrap-around label.
According to the FDA, Sunstar is notifying its direct distributors and customers by U.S. Postal Service priority mail and is arranging for the return of all recalled products. Patients, pharmacies and health care facilities that possess the products should stop using and dispensing them immediately.
Dental offices that have prescribed the oral rinse to patients are encouraged to notify them of the recall and advise them to stop using the product.
Dentists with questions about the recall can contact Sunstar Americas Inc. at 800.528.8537 or by email at [email protected] Monday-Friday from 8 a.m. to 5 p.m. CST.